China’s Sinopharm Seeking Approval for COVID-19 Vaccine
Sinopharm, a Chinese pharmaceutical company that claims to have inoculated over 1 million people with its experimental COVID-19 vaccines, has applied for a market approval, state-run media outlet Xinhua Finance reported Wednesday.
The Beijing-based company has two vaccine candidates in late-stage human trials, and it’s unclear whether one or both has been submitted for approval. China’s National Medical Products Administration, the country’s regulatory body for pharmaceuticals, had not publicly commented by time of publication.
According to domestic and international clinical trial registries, Sinopharm has not yet completed its stage 3 human trials, conducted in countries like Peru, Morocco, Egypt, Argentina, and the United Arab Emirates.
Sinopharm also has not released any preliminary data from its stage 3 trials, although Chinese authorities authorized both experimental vaccines for emergency use in July. Liu Jingzhen, the company’s CEO, said nearly 1 million people have since been inoculated. “(We) have not received reports of a single case suffering a serious adverse event, only mild symptoms,” he told domestic media.
In July, China’s National Medical Products Administration announced a new set of guidelines, aiming to fast-track drug review and approval processes. The regulation specifically said “breakthrough pharmaceuticals” and products that “prevent and treat major infectious diseases” would qualify for an expedited approval process, even if they had not completed late-stage human trials.
Editor: David Paulk.
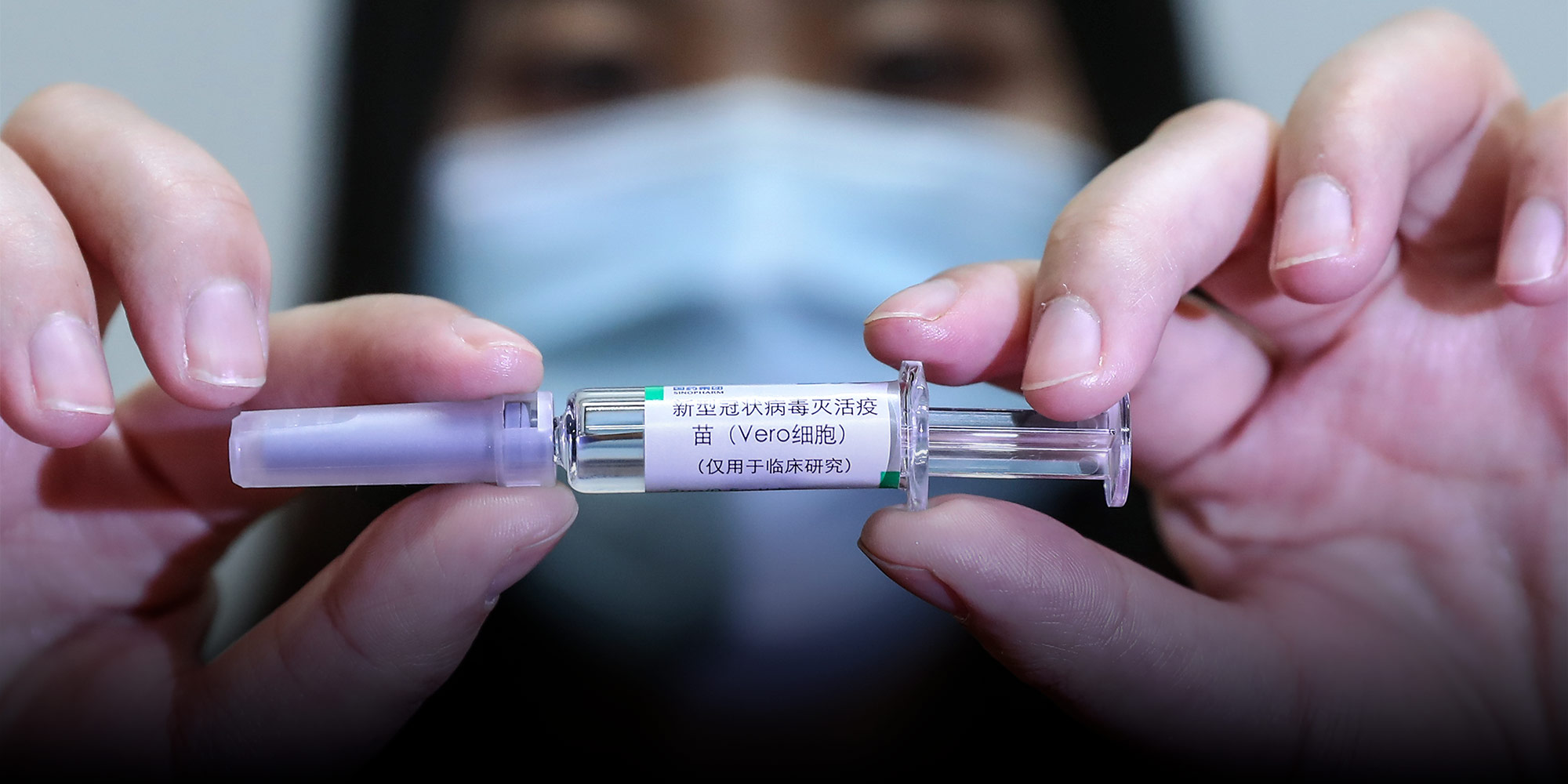
(Header image: A staff member shows a Sinopharm-developed coronavirus vaccine candidate in Beijing, April 2020. Zhang Yuwei/Xinhua)