China Mulls Vaccinating Children and Teenagers Against COVID-19
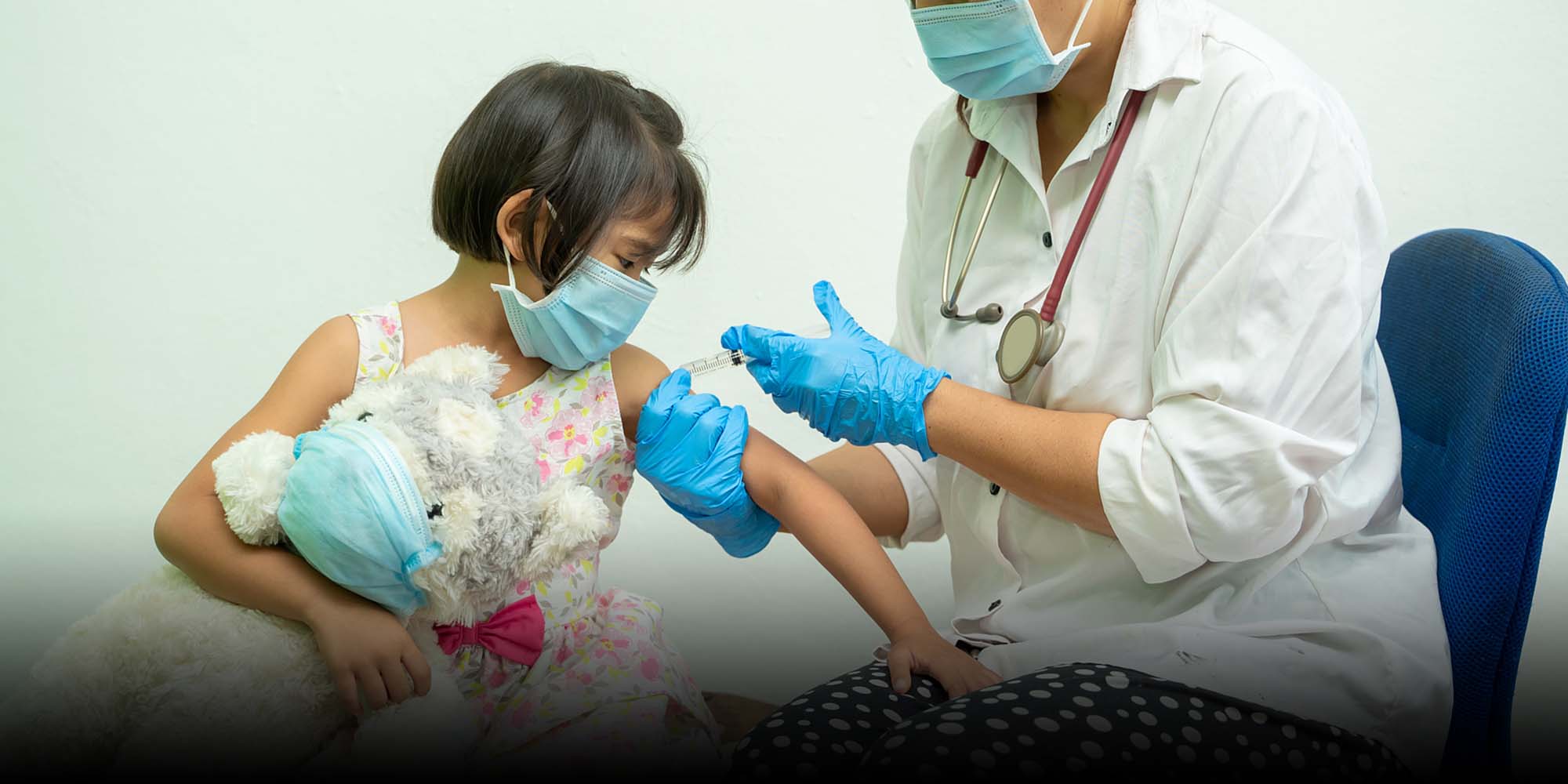
China is considering giving COVID-19 vaccines to children and adolescents — despite the country having not yet released detailed data involving the target population — as the country ramps up its vaccination drive amid sporadic outbreaks that have put authorities on high alert.
Feng Zijian, a deputy director-general at the Chinese Center for Disease Control and Prevention, said authorities were now working on the next phase of inoculating the under-18 population without giving a specific timeline, domestic media reported. Currently, China’s inoculation drive mostly involves people aged 18 to 59, with some cities vaccinating those over 60.
“Right now, the government is very seriously, actively, and cautiously researching the next stage of its inoculation plan, policies, and services,” Feng said during a conference held in the central Chinese city of Wuhan on Wednesday, adding that the authority would start inoculating adolescents and children, after the elderly.
While COVID-19 vaccines developed in China haven’t been granted approval for adolescent use, the United States has authorized its Pfizer vaccines for use in those aged 12-15.
Feng’s remarks come as China faces a fresh outbreak in the southern Guangdong province. As of Thursday, the local health authority had reported over 50 locally transmitted COVID-19 cases since the outbreak began late May.
Research suggests children under 14 are less likely to become seriously ill with COVID-19, but the group makes up about 18% of China’s total population. They are an essential demographic for the country to achieve herd immunity, a scenario where at least 70% of the population has become immune to the virus either through infection or vaccination based on current estimations.
By Thursday, China had administered over 700 million doses of COVID-19 vaccines, with less than 20% of the population fully vaccinated, according to Zhang Wenhong, an infectious disease specialist heading Shanghai’s coronavirus response.
State-owned pharmaceutical companies Sinovac and Sinopharm said that their vaccines — which are now approved by the World Health Organization for emergency use in those aged 18 and up — have shown to be safe in children and can elicit immune responses.
A small-scale clinical trial of the vaccine in 500 children aged 3-17 showed the vaccine to be safe and capable of eliciting an immune response, a researcher with Sinovac said during a conference in March, without releasing detailed data. Meanwhile, Sinopharm’s chairman told state-run Xinhua in January that they have “solid clinical trial data that shows (the COVID-19 vaccine) can be given to people from 3 to 17 years old with no safety issues.”
Editor: Bibek Bhandari.
(Header image: People Visual)