In China, New Measures to Close Gap in Rare Disease Treatment
An increasing number of drugs for rare diseases have been introduced in the market over the last year, signaling China’s efforts to align with global standards in rare disease treatment, a new report from the Illness Challenge Foundation and Frost & Sullivan indicates.
The report, released to mark World Rare Disease Day, observed every year on the last day of February, also underscored continuing challenges such as the high costs and difficulties in importing new medications.
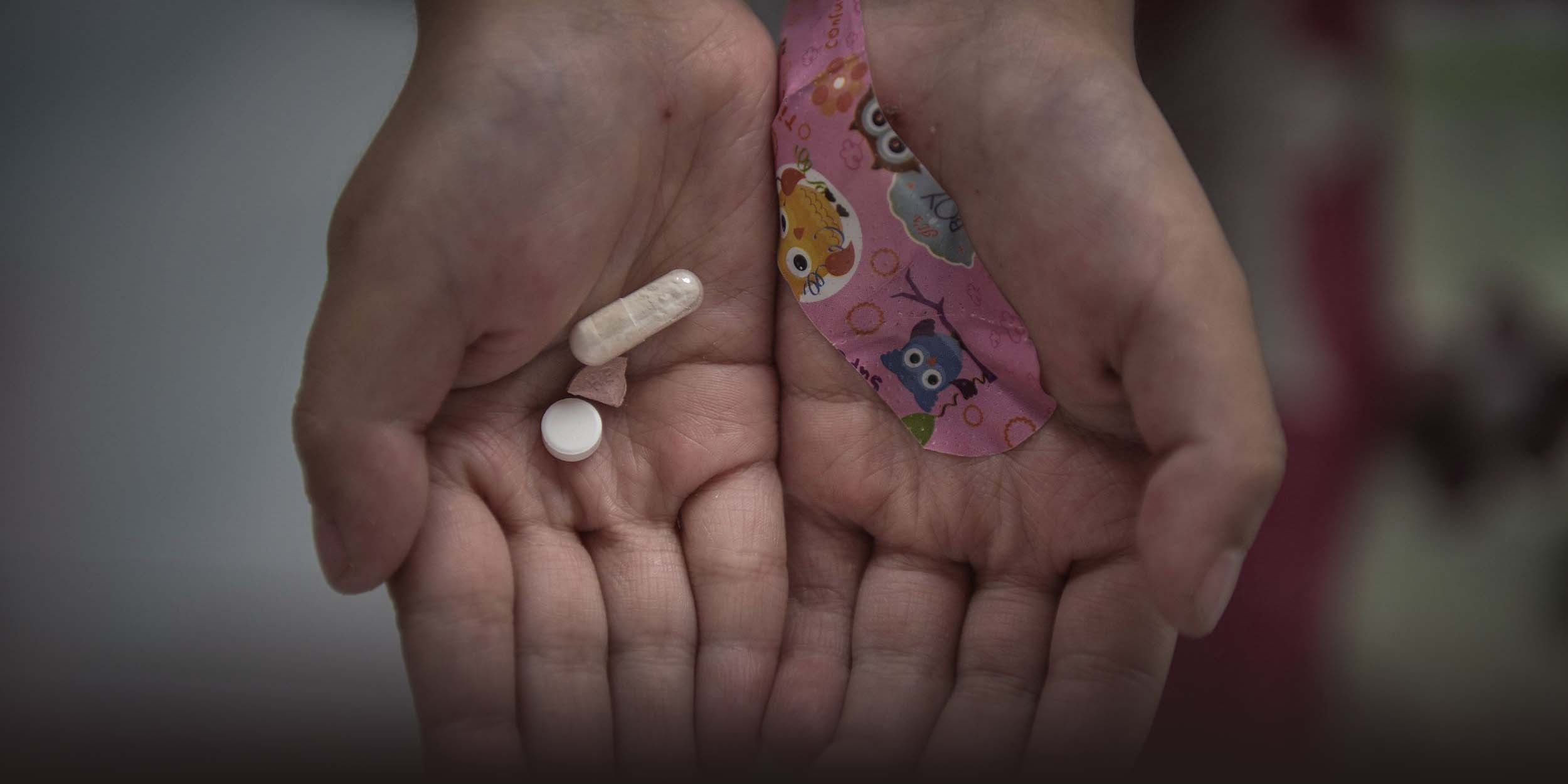
An estimated 20 million individuals are affected by rare diseases in the country. They include Gaucher’s disease, an inherited metabolic disorder leading to organ and tissue damage; Moyamoya disease, a genetic blood vessel disorder that can cause strokes; and Osteogenesis Imperfecta, also known as “porcelain doll disease” in Chinese, which causes bones to become extremely brittle and prone to breakage even with minimal force.
As of February 2023, 199 drugs addressing 87 rare diseases were launched worldwide, with China introducing 103 drugs covering 47 rare diseases, just half the global rate.
According to the report, China has since initiated a special process for the expedited review of critically needed foreign drugs. They include orphan drugs, which are specifically developed to treat rare medical conditions but, due to the limited number of patients, would not be commercially viable without government incentives.
The effort has resulted in 15 orphan drugs receiving marketing approval through a priority review in 2023, increasing from just three in 2018. And by the end of the year, China had made available 165 types of rare disease drugs, addressing 92 different rare diseases.
However, the latest list from the Center for Drug Evaluation under the National Medical Products Administration has identified 73 types of urgently needed overseas drugs, with half targeting rare diseases. Yet, only 22 of these have been introduced into the Chinese market.
Experts warn that the gap in the domestic drug supply may drive patients to illegally import medications from abroad, often without proper medical supervision. In 2021, a man from the central Henan province was accused of “drug trafficking” for smuggling Clonazepam, a psychotropic drug used for a rare form of epilepsy that can be addictive with long-term use, for his child.
In response, the government initiated a temporary import scheme for the drug in June 2022 and has since sought to accelerate importing more orphan drugs.
According to Wang Yi’ou, founder of the Illness Challenge Foundation, the absence of a dedicated system to cover the high costs of rare disease treatments also posed significant hurdles.
“Many companies believe patients won’t be able to afford these treatments even if they are introduced. Solving the payment issue for rare diseases would encourage more foreign drugs to enter the market and motivate domestic companies to develop new treatments,” explained Wang.
Echoing similar concerns, Yu Lei, head of pharma giant Sanofi’s rare diseases division in China, said: “(Orphan drugs) priced at 2 million yuan ($278,000) are not a viable option for the majority, particularly if the treatment is lifelong,” he said.
To ensure treatment is more accessible, authorities have included 15 new rare disease drugs under medical insurance schemes. And to further expedite the introduction of overseas drugs and medical devices, special trial zones have been rolled out to facilitate the import of rare disease drugs not yet registered or available domestically.
Contribution: Lü Xiaoxi; editor: Apurva.
(Header image: VCG)